|
Following US midterm elections held earlier this month, MRP highlighted the little-changed fortunes of the cannabis industry, based on a new composition of congress and a couple of states approving the sale of marijuana for recreational use as part of local ballot initiatives. However, a similar level of success achieved by the budding psychedelics industry is likely to be more impactful on the outlook for that space.
As part of a groundbreaking initiative in Colorado, a majority of the state’s voters approved Proposition 122, therefore choosing to join Oregon as the second state in the US to decriminalize psilocybin and other hallucinogenic mushrooms, establishing a regulated industry for plant-based psychedelic drugs. By By late 2024, Axios reports licensed “healing centers” can begin providing the hallucinogenic psilocybin and psilocin to clients.
Though Oregon already decriminalized psilocybin in 2020, the state ballot for their 2022 elections allowed certain counties to “opt out” of psilocybin sales and use – which is what 25 of Oregon’s 36 counties (69%) chose to do. That doesn’t mean much for the opening of psilocybin clinics in the state, which will begin in 2023, but these clinics can only exist in 11 counties. Per Leafly, county and city-level restrictions on such enterprises are par for the course in the US. For example, 62% of counties in California do not allow the sale or possession of cannabis despite it being legal at the state level.
The development of permissive regulatory frameworks at the state level come as more and more evidence attests to the therapeutic and anxiety fighting properties of natural hallucinogens. As such, MRP has highlighted psychedelics several times throughout the past couple of years. In particular, we first noted their ability to treat depression, anxiety, and post-traumatic stress disorders in May 2021. Around that time, trial results across several studies had begun to prove the medicinal value of LSD, MDMA and psilocybin is even greater than previously thought.
A paper from the Multidisciplinary Association for Psychedelic Studies, cited by Forbes, found that 67% of participants who received three treatments of MDMA assisted therapy no longer qualified for a PTSD diagnosis, while 88% experienced a clinically significant reduction in symptoms. The study recruited 90 PTSD patients and was done over an 18-week period.
A newer study, produced earlier this year by Johns Hopkins Medicine and published in the Journal of Psychopharmacology, found that substantial antidepressant effects of psilocybin-assisted therapy may be durable for at least a year following acute intervention in some patients. Treatment response and remission among 27 patients aged 21–75 with moderate to severe unipolar depression were 75% and 58%, respectively, at 12 months and there were no serious adverse events judged to be related to psilocybin in the long-term follow-up period.
The Wall Street Journal notes the Food and Drug Administration (FDA) approved the use of a drug similar to ketamine for treatment-resistant depression several years ago, and is expected to rule on an application for MDMA-assisted therapy in 2023. Meanwhile, the New York Times forecasts psilocybin to receive approval for depression from the FDA by the end of the decade.
More recently, results from the largest and most rigorous clinical trial of psilocybin to date showed that patients who received a single dose of 25mg COMP360 psilocybin experienced a highly statistically and clinically significant rapid reduction in symptoms of depression compared to 1mg at 3 weeks. The trial, which included 233 people with treatment-resistant depression (TRD) across 10 countries, found 37% of people with TRD in the 25mg group met criteria for response at week 3 (≥50% decrease in depressive symptoms). More significantly, ScienceDaily notes that 20% met criteria for sustained response at week 12.
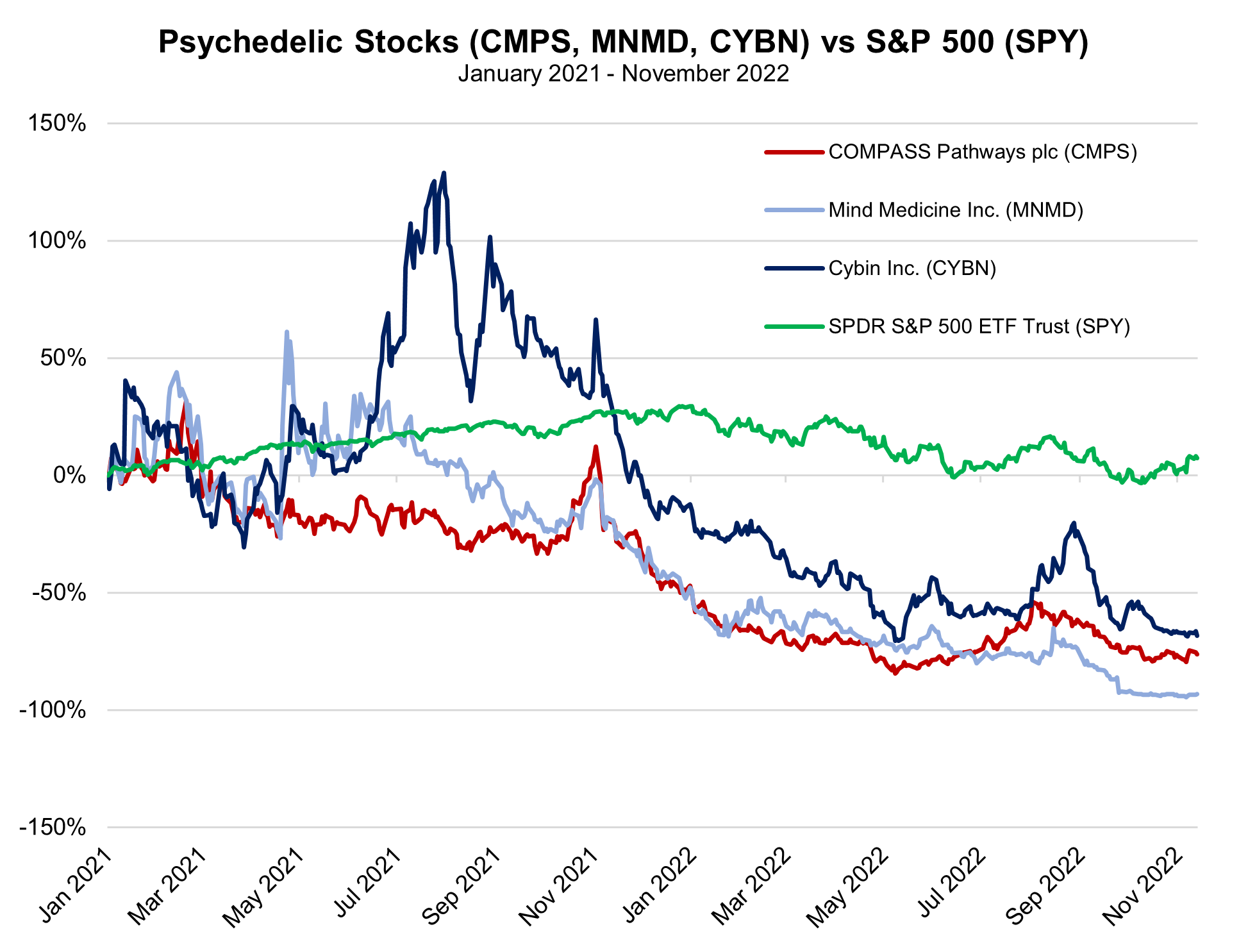
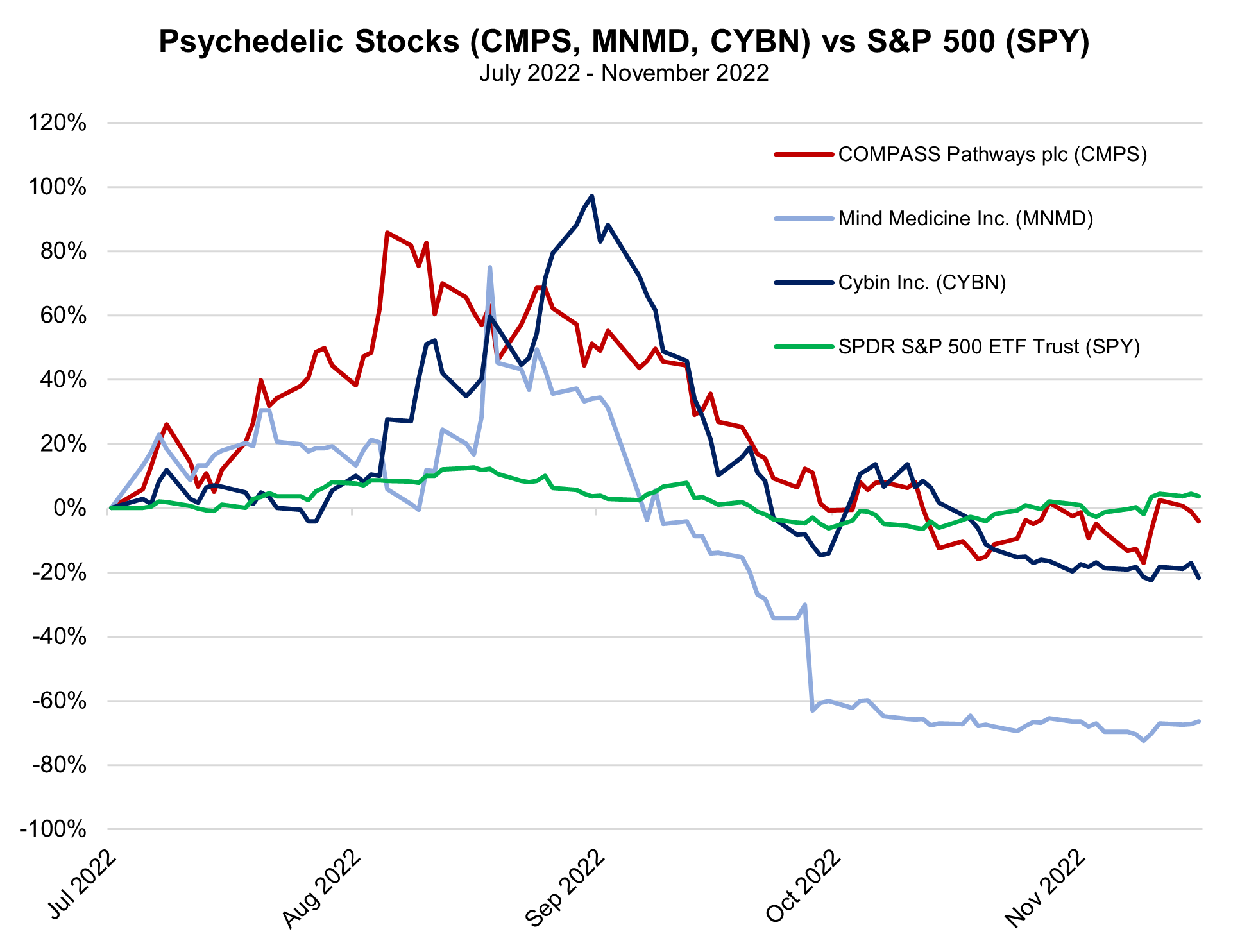
This phase 2B trial, published in The New England Journal of Medicine, was sponsored by publicly traded COMPASS Pathways PLC, which is a mental health care company focused on the use of innovative therapies, including psilocybin use, to treat depression. The ongoing research will soon expand into the world’s first phase 3 trial of psilocybin, which will include two clinical trials, testing of multiple dosages instead of a single dose, as well as a long-term follow-up that could enroll nearly 1,000 patients. With early data expected in 2024, New Atlas reports COMPASS will look toward FDA approval for their treatments by the end of 2025. COMPASS maintains a cash position of $173.1 million and a current market capitalization of $440.7 million.
The addressable market for psychedelic products and therapies is vast. In 2019, roughly 51.5 million adults in the United States were living with some form of mental illness. In the first year of the COVID-19 pandemic, global prevalence of anxiety and depression increased by a massive 25%, according to a scientific brief released by the World Health Organization (WHO).
Several publicly traded entities, including Mind Medicine (MindMed) Inc. (MNMD), COMPASS Pathways plc (CMPS), Atai Life Sciences (ATAI), and Cybin Inc. (CYBN), are currently available for most investors. However, shares of firms in the psychedelic space have been slammed over the last year by a broad downturn in equities. |



Leave a Reply